Documents with modest margins and no spaces concerning paragraphs and headings is often hard to evaluate, tough and slower to read. Room the contents out so that the kind/font is straightforward to read through for all people.
Skilled folks knowledgeable in production and quality Regulate needs to be to blame for the written content and distribution within the organization of Directions and learn formulae. These need to be duly signed and dated.
Raw content testing: This vital action entails analyzing the ingredients used in production pharmaceutical goods. It makes sure that raw resources meet the necessary requirements and are free of charge from impurities or contaminants that would compromise the standard of the final product.
Exactly where the amount isn't set, the calculation for every batch sizing or charge of production need to be bundled. Versions to portions should be involved wherever justified
This really is an open up-access article dispersed underneath the phrases on the Innovative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, delivered the first work is effectively cited.
External documents necessary for quality management has to be determined and managed, and documents need to be shielded from unauthorized improvements.
Supplier qualification documents depth the qualifications and read more approvals of suppliers to comply with relevant specifications. These documents are useful for examining the performance of suppliers for guided choices on renewal/termination for outsourced expert services.
Revalidation is needed to be sure that any changes designed in the procedure ecosystem, whether or not finished intentionally or unintentionally, will not adversely have an impact on the process characteristics and product or service quality.
Completed product or service testing: QC pros inspect and approve solutions to be certain they fulfill the required buyer and regulatory more info benchmarks.
These records must be numbered with a singular batch or identification selection and dated and signed when issued. In continuous production, the solution code along with the day and time can function the unique identifier until finally the final variety is allotted.
Day may be recorded by electromagnetic or photographic suggests, but comprehensive techniques referring to whatever procedure is adopted need to be obtainable. Precision on the history ought to be checked as per the described technique.
In the food, drug, and medical unit industry it can be vital that great techniques are set up to ensure a managed and dependable performance; it is an essential Element of GMP. Strategies should be clear, concise, and sensible.
With decades of working experience in regulatory compliance, JAF Consulting is really a trustworthy companion for pharmaceutical companies around the world.
Usually use one strike define (For example Incorrect Entry) to mark the incorrect entry in this type of method that entry continues to be readable.
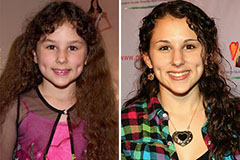 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now!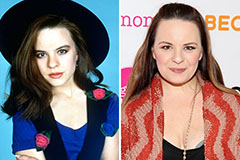 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! David Faustino Then & Now!
David Faustino Then & Now! Katey Sagal Then & Now!
Katey Sagal Then & Now! Naomi Grossman Then & Now!
Naomi Grossman Then & Now!